Neurodegenerative
diseases

Early diagnosis is the key to successful treatment
In the competence field of biospectroscopy, innovative applications of infrared (IR) spectroscopy are being developed for the early diagnosis of neurodegenerative and neurological diseases. For this we examine body fluids such as blood plasma and cerebrospinal fluid using attenuated total reflection (ATR) spectroscopy. The immuno-infrared sensor developed by us for the prediction of Alzheimer’s disease (Nabers et al, EMBO Molecular Medicine 2018) deserves special mention.
Currently, Alzheimer’s disease is only recognised when clinical symptoms appear. At this stage of the disease, however, the brain is already irreversibly damaged. Detection at very early stages, even before clinical symptoms appear, is essential for successful treatment. Currently, the clinical diagnosis of Alzheimer’s disease is primarily based on neuropsychological assessments. In addition, neurochemical biomarker concentrations in the spinal fluid are determined. In exceptional cases, the nuclear medical method of positron emission tomography (PET scan) is used to detect amyloid plaques in the brain. This method is an imaging procedure which, although accurate, is associated with the administration of a slightly radioactive substance and high costs per analysis.
Development of a blood test for early detection of Alzheimer’s disease
Enormous efforts are being made worldwide to develop non-invasive, cost-effective and sensitive diagnostic tools. Therefore, one focus of the research work in ProDi is directed towards the development of a blood test for the early detection of Alzheimer’s disease. In the competence area of biospectroscopy, we use our expertise in spectroscopic methods for this purpose.
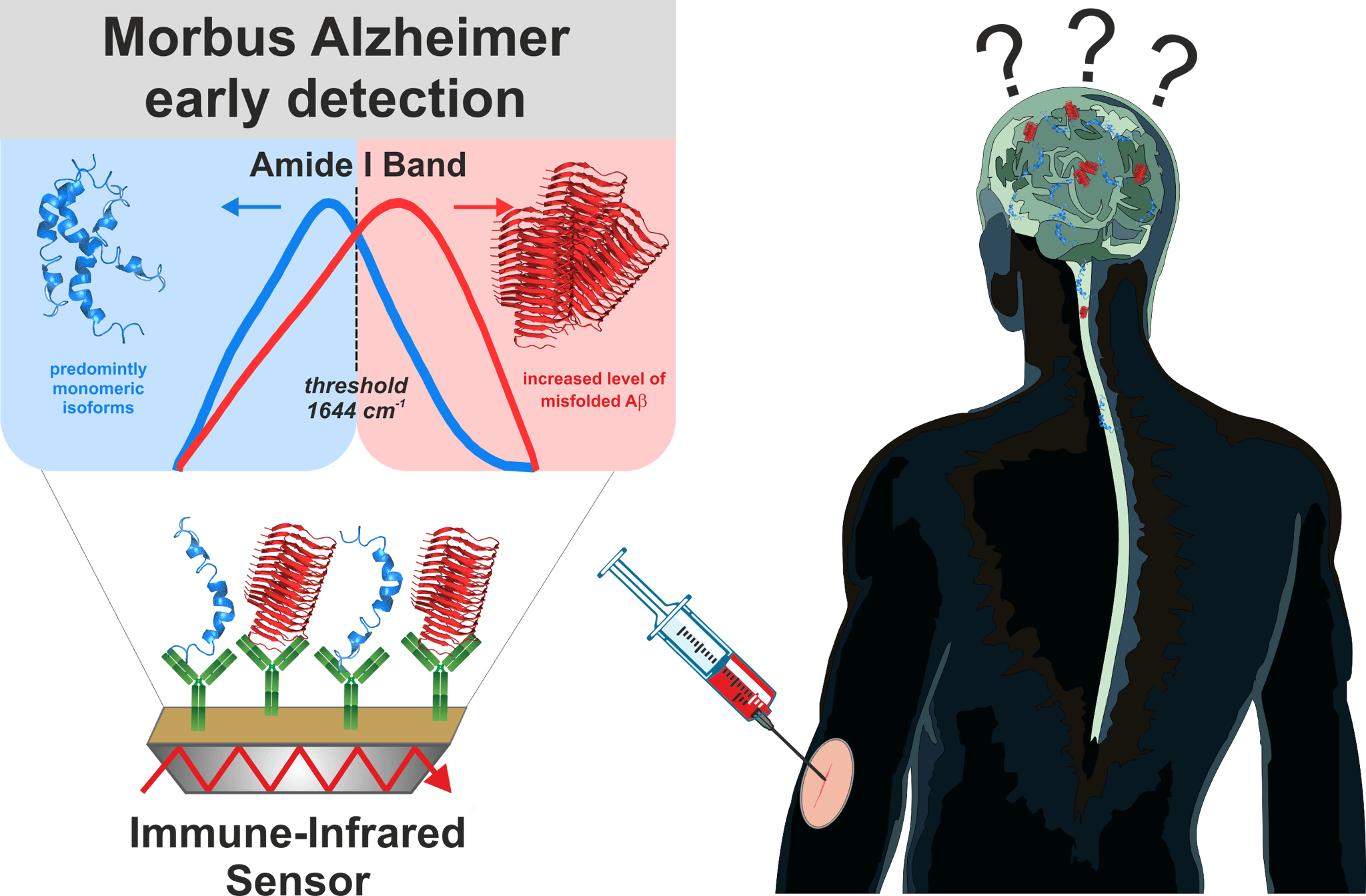
Figure 1: Alzheimer’s disease: early diagnosis by spectroscopic analysis of body fluids using the immuno-infrared sensor
Starting point for early diagnosis in blood: Amyloid-ß plaques
One possible cause of Alzheimer’s disease is the refolding of the amyloid-ß (Aß) peptide from a disordered mixture of partially unfolded and α-helical secondary structures to ordered, aggregated pathogenic complexes of many Aß peptides, the ß-folded fibrils. These fibrils form large aggregates that are responsible for the plaque deposits in the brain associated with Alzheimer’s disease. The process of protein refolding starts about 15-20 years before clinical symptoms appear and the disease becomes clinically evident. The increased concentration of Aß fibrils in the brain also leads to an increased concentration of Aß aggregates in the spinal fluid and blood. The ratio of disordered Aß peptides to ordered Aß aggregates provides us with a starting point for spectroscopic analysis of blood plasma but also of spinal cord and other body fluids.
Immuno-infrared sensor: diagnosis by spectroscopic body fluid analysis
The immuno-infrared sensor developed by us and awarded the Inventor’s Prize Prize detects the secondary structure distribution of Aß-peptides in body fluid samples. From the secondary structure distribution, the ratio of ordered and disordered Aß-peptides is determined, which allows a differentiation between samples from Alzheimer’s and non-Alzheimer’s patients. Initially, we have used the method in various studies to analyse spinal fluid (cerebrospinal fluid). We achieved a sensitivity of 94% and a specificity of 88% in differentiating between Alzheimer patients and control subjects (Nabers et al Journal of Biophotonics 2016). This means that we recorded 94% of patients with Alzheimer’s disease as safe as sick and 88% of actually healthy patients as healthy.

Figure 2: Connecting a blood plasma sample to the immuno-infrared sensor
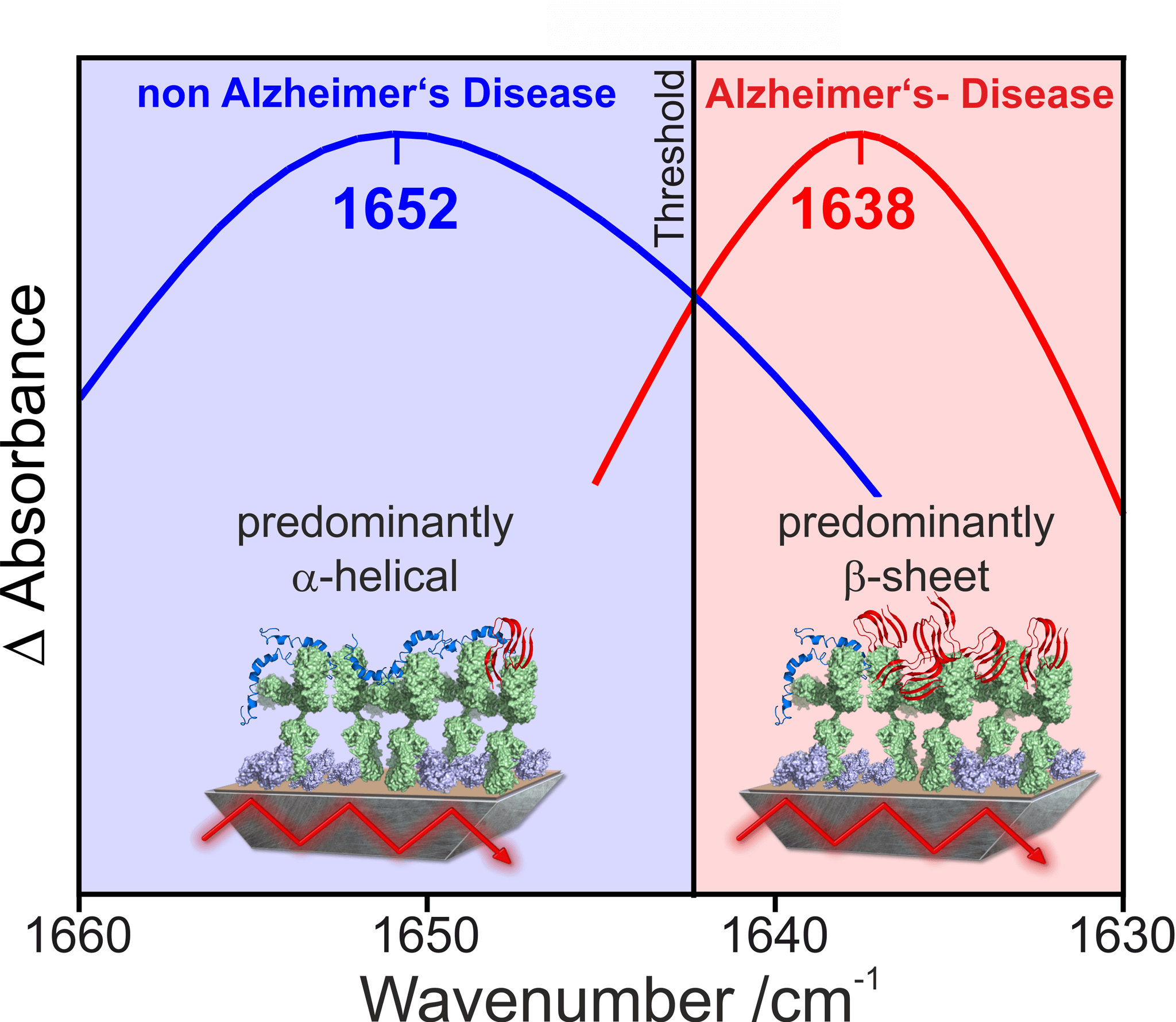
Figure 3: Spectral discrimination of Alzheimer’s disease and non-Alzheimer’s disease patients
Early diagnosis through spectroscopic analysis of blood plasma
Since the collection of spinal fluid is risky and painful, we have extended our diagnostic approach to the spectroscopic analysis of blood plasma. Blood plasma is obtained through the minimally invasive procedure of blood sampling. When analysing blood plasma, we were able to achieve a sensitivity of 75% in the first step and a specificity of 88% in well confirmed Alzheimer’s cases (Nabers et al Analytical Chemistry 179). In the next step, we demonstrated the early diagnostic potential of the immuno-infrared sensor by means of long-term studies. With our diagnostic method we achieved a preclinical detection of Alzheimer’s disease in blood plasma with a sensitivity of 71% and specificity of 91%. Our study shows that the immuno-infrared sensor detects Alzheimer’s disease in blood plasma 8-14 years before clinical symptoms occur, and thus at a very early stage of the disease (Nabers et al EMBO Molecular Medicine 2018). Combining our structural biomarker to predict future cognitive impairment with genetic risk (APOE status), our method has accurately predicted in 86% of cases which study participants (ESTHER study Heidelberg) developed Alzheimer’s symptoms within 8-14 years after sampling (Stocker et al Alzheimer’s & Dementia 2019.
A basis for diagnostic tests of various neurological diseases
In addition to the Aß-peptide, there are other proteins whose alteration of the secondary structure has been linked to diseases. These proteins are known as biomarkers. The Tau protein, for example, is another biomarker for Alzheimer’s disease. Other structure-altered proteins that act as biomarkers are alpha-synuclein or huntingtin, which are associated with Parkinson’s symptoms or Huntington’s disease, respectively. The analysis of the secondary structure of proteins with the immuno-infrared sensor thus also offers the possibility of investigating other diseases more closely. A safe and simple blood test for early diagnosis is a big step towards therapy and prevention of neurodegenerative diseases.
Press
Press releases
Press review
External Funding

111.08.03.05-133974
Validation and standardization of the protein diagnostics workflow

Correlation between Aβ42 decrease and misfolding in blood
PURE – Protein Research Unit Ruhr within Europe
Validation of marker-free imaging methods and newly identified biomarkers using the PURE consortium