Experimentelle Onkologie
Research Focus
Die ColoPredict Plus 2.0 Register Study

The ColoPredict Plus 2.0 registry study The Colopredict Plus 2.0 molecular registry – a platform for target identification and for precision oncology in early colon carcinoma.
Through the close cooperation between the affiliated oncology clinics and the Institute of Pathology, structures have been created in recent years that make a significant contribution to the translation of research into clinical projects.
The central project of clinical and translational research is the non-interventional, multicentre Colopredict Plus 2.0 registry study.
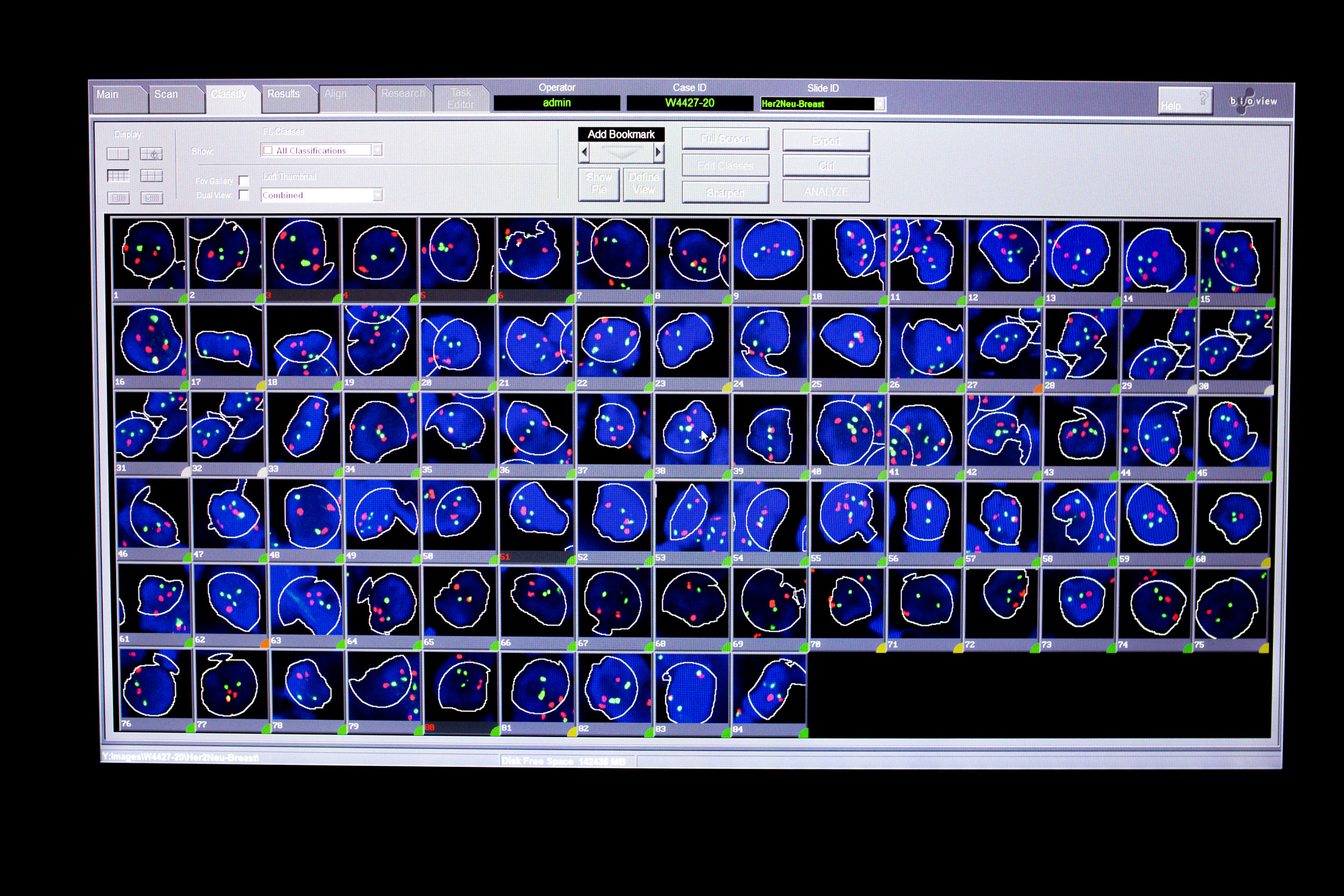
PanDa DETECT
BioNETRUB
Research questions often arise due to the progress of analytical methods or on the basis of new findings in the field of medicine. So-called biobanks or biomaterial banks are indispensable for examining questions, e.g. on very specific tumours (e.g. with special molecular characteristics).
We are therefore endeavouring to collect tissue and blood samples from patients with cancer or patients at increased risk of developing such a disease in the interdisciplinary biobank network of the Medical Faculty of the Ruhr University Bochum (BioNetRUB) and distribute them to promising medical research projects.
The aim is to improve prediction, prevention, diagnosis and therapy in human medicine. In addition, the collected samples and data can also be used to research fundamental mechanisms for understanding diseases.
CancerCovid
CancerCOVID – Resource allocation for cancer medicine in the context of Sars-CoV-2
The pandemic triggered by the novel SARS-CoV-2 poses unprecedented challenges for the German health system. There are currently no effective therapies to treat the lung disease Covid-19 caused by SARS-CoV-2.
Clinical Studies (ITZ-Ruhr)
Oncology is also involved in clinical trials. Register studies, physician-initiated studies according to the AMG and studies commissioned by the pharmaceutical industry are carried out and supported. The aim is to give patients access to optimised therapy procedures and to improve evidence-based treatment.
A link to the studies conducted at the ITZ-Ruhr can be found here:
COVID-19-Biomaterial Bank
To establish a prospective collection of biomaterial and associated clinical and demographic data from patients with SARS-CoV-2 infection and associated controls, with particular reference to immunocompromised cohorts.
The primary aim of this project is the clinical and serological characterisation of patients with SARS-CoV-2 infection and COVID-19, respectively, with special emphasis on immunosuppressed patients and non-immunocompromised controls. Clinical prognostic factors and biomarkers for a severe course (e.g. cellular and humoral immunodeficiencies) will be identified for the collective. Secondarily, control collectives of non-ill patients who are at increased risk of SARS-CoV-2 infection will be investigated to the same extent, also with regard to possible indications of a previous infection.
A special focus is placed on oncological patients and questions such as those questions such as the vaccination response of this group.
Employees
Dr. med. Celine Lugnier
Dr. med. univ. Oliver Overheu
Dr. med. David Witte
Publications
Here you will find current publications:
Gallery
Vacancies
For doctoral theses please contact
Dr. med. Anna-Lena Kraeft
E-Mail: anna-lena.kraeft@klinikum-bochum.de